Participants : P. Allongue and F. Maroun
PhD student : A. Damian (2009)
This study aims at comparing the adsorption energy of Ni adatoms on different surfaces. Because this difference is expected to be very small, it is not accurate to compare experiments conducted on different substrates. In this work, we use a bimetallic PdAu(111) instead, which is a Au(111) surface covered by electrodeposition with ½ monolayer of Pd (Fig. 1a). From in situ STM observations, we derived the Ni coverages on Au and Pd. Fig. 1b demonstrates that Ni deposition starts on gold and then continues on the Pd islands until full surface coverage is achieved. This selectivity disappears however for the 2nd Ni monolayer. To establish that the above selectivity originates from a difference in interaction energy Ni-Au and Ni-Pd, we have precisely determined the dissolution threshold potential using in situ STM. Images (c-d) demonstrate that Ni dissolution potential is 40 mV more negative on Pd than on Au, which translates into a difference in interaction energy of 80 meV, Ni being less strongly bonded to Pd.
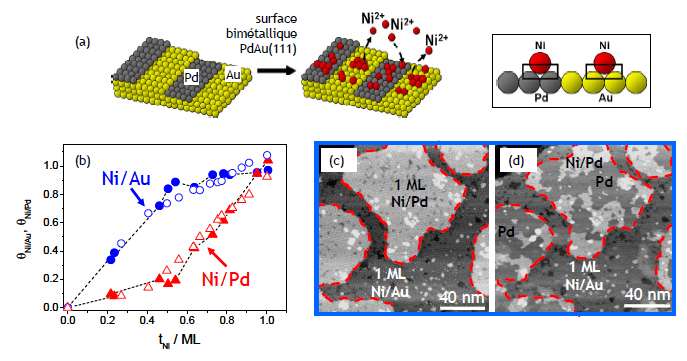
Figure 1 : (a) Concept of the experiment to compare the interaction energies Ni/Au and Ni/Pd; (b) Variations of the Ni coverage on Au and Pd during the formation of the 1st Ni monolayer. These plots mean that Ni deposition is faster on Au than on Pd. This difference disappears for the 2nd Ni ML. (c-d) In situ STM images showing that Ni is selective. It starts from Pd islands.
Publications :
– A. Damian, F. Maroun, and P. Allongue, "Selective Growth and Dissolution of Ni on a PdAu Bimetallic Surface by In Situ STM: Determining the Relative Adsorbate-Substrate Interaction Energy," Phys. Rev. Lett. 102 (19), 196101 (2009).
– A. Damian, F. Maroun, and P. Allongue, "Electrochemical growth and dissolution of Ni on bimetallic Pd/Au(1 1 1) substrates," Electrochim. Acta 55 (27), 8087-8099 (2010).

