INTRODUCTION
Solidification microstructures that form under steady-state growth conditions (cells, dendrites, eutectic, etc.) are reasonably well understood in comparison to other, more complex microstructures, which form under intrinsically non-steady-state growth conditions where the evolution of microstructure is controlled by the competition between the nucleation and growth of several phases. Some important examples in this latter class include microstructures forming in peritectic systems [1-11], in highly undercooled droplets [12, 13], and in strip cast stainless steels [14]. Prediction of phase and microstructure selection in these systems has been traditionally based on heterogeneous nucleation on a static interface or by comparing the relative growth rate of different phases/microstructures under steady-state growth conditions. The formation of new phases occurs via nucleation on or ahead of the moving interface. In addition, the actual selection process is controlled by a complex interaction between the nucleation process and the growth competition between the nuclei and the pre-existing phase under non-steady-state conditions. As a result, it is often difficult to predict which microstructure will form and which phase will be selected under prescribed processing conditions.
This paper addresses this critical role of nucleation at moving boundaries in the selection of phases and microstructures through quantitative experiments and numerical modeling in peritectic systems. In order to create a well characterized system to study this problem, we will focus on to the directional solidification of binary alloys with compositions in the two phase region of the peritectic phase diagram. Furthermore, we shall impose a large enough ratio of temperature gradient/growth rate to suppress the morphological instability of both the parent (a) and the peritectic (b) phases, i.e. each phase alone would grow as a planar front. Our combined experimental and theoretical results show that, even in this simplified case, the growth competition of these two phases leads to a rich variety of microstructures that depend sensitively upon the relative importance of nucleation, diffusion and convection [8-10].

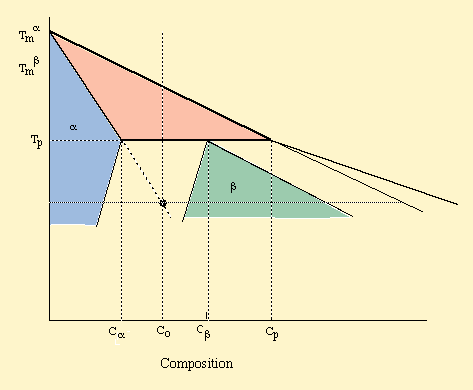
Fig. 1. A schematic diagram of peritectic system showing the primary phase, a, and the peritectic phase, b.
Figure 1 shows schematically a typical peritectic phase diagram in which solidus and liquidus lines have been assumed to have constant slope to simplify the analysis. a, b and L represent the primary solid phase, peritectic solid phase and the liquid phase, respectively. Tp is the peritectic temperature at which a, b and liquid phases can coexist in equilibrium at the corresponding compositions of Ca , Cb and Cp. The composition of the liquid Cp is usually called the Peritectic Composition though Peritectic point has also been used to refer to Cb [1]. The range (Ca £ C £ Cp) is the Peritectic Range which is subdivided into two regions as Hypoperitectic (Ca £ C £ Cb ) and Hyperperitectic (Cb £ C £ CP). During directional solidification of an alloy with initial homogeneous melt composition C0 (< Cp) , the primary a phase solidifies first, followed by the formation of the peritectic phase (b). The interactions between the primary and the peritectic phase can lead to a variety of microstructures. By varying the processing conditions (temperature gradient, pulling velocity, initial alloy composition and sample diameter), a wide spectrum of complex microstructures have been observed, as shown in Fig. 2. These microstructures can be broadly classified into the following groups: (a) discrete bands, (b) partial bands, (c) single band, (d) oscillating structures, (e) coupled growth, and (f) dispersed phase through the nucleation ahead of the interface. In this paper we shall concentrate only on the formation of bands and oscillating microstructures.
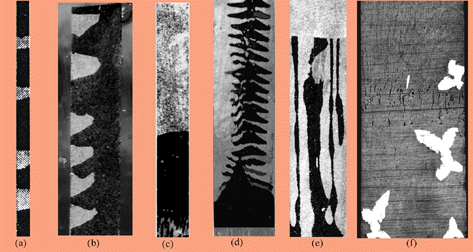
Fig. 2. Different microstructures observed in the two phase region of peritectic systems. (a) Discrete diffusive band formation in Sn- 0.9 wt % Cd alloy, V=3.0 µm/s, G = 23 K/mm, d = 0.6 mm (b) Partial diffusive bands in Sn- 0.75 wt % Cd alloy, V= 4.0 µm/s, G = 23 K/mm, d = 1.0 mm (c) A single a to b transition in Sn- 1.3 wt % Cd alloy, V= 3.0 µm/s, G = 23 K/mm, d=0.6 mm (d) A continuous a and b structures in Sn- 1.3 wt % Cd alloy, V= 3.0 µm/s, G = 23 K/mm, d = 6.0 mm. (e) A coupled growth of a and b phases in Sn- 0.75 wt % Cd alloy, V= 1.0 µm/s, G = 13.5 K/mm, d = 2 mm. (f) Nucleation of b -phase ahead of the a:liquid interface in the Sn- 13.0 wt % Sb alloy, d = 1.0 mm.
Recent experimental studies have shown that diffusion as well as convection play crucial roles in establishing the variety of two-phase microstructures observed in peritectic systems. These experimental results have been coupled with analytical and numerical models which are based on: (a) diffusive growth [6, 8] (b) strong convective growth which can be described by the boundary layer model [8], and (c) steady-state or oscillating convection in the liquid [10]. These models have offered an insight into the inherent oscillatory dynamics of repeated nucleation and growth of two phase solids or oscillatory but continuous growth of the two phases. We shall first describe recent experimental results, and then discuss different theoretical models that quantitatively reproduce a rich variety of microstructures in peritectic systems.