Participant : F. Maroun
PhD students : Mathilde Bouvier (2021), Ivan Pacheco Bubi (2021)
In this theme, we are interested in energy conversion by water splitting. To do this, we are studying the electrochemical synthesis of model thin layers of transition metal oxides (Co, Fe) to understand their catalytic property in the decomposition of water into oxygen. We also study the structure and redox state of the surface in operando. This allowed us to show that the Co3O4 layers transform on the surface into CoOOH during the water decomposition reaction. This transformation is reversible and explains why the catalytic properties of Co3O4 and CoOOH are similar.
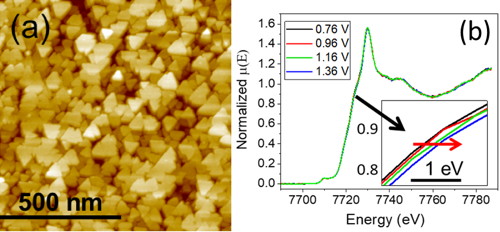
Figure : (a) image by atomic force microscopy of a layer of Co3O4 electrodeposited on Au. (b) XANES spectra of a layer of Co3O4 measured in operando as a function of potential.
Publications:
[1] Reikowski et al., ACS Catalysis, 2019, 9, 3811-3822
[2] Wiegmann et al., ACS Catalysis, 2022, 12, 3256-3268
[3] Bouvier et al., ACS Appl. Energy Mater. 2023, 6, 14, 7335–7345

